October 11, 2024
3 min read
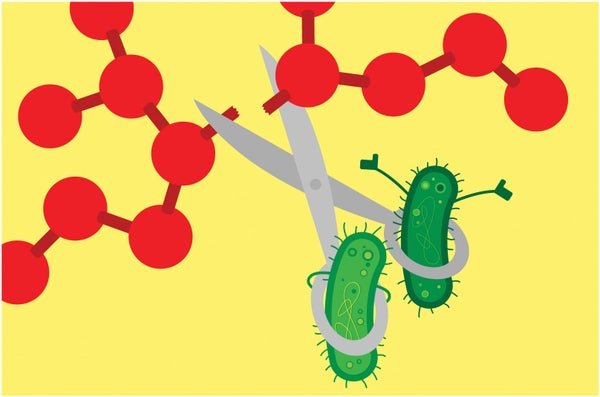
Enlisting Microbes to Break Down ‘Forever Chemicals’
Bacteria can degrade particularly tough PFAS varieties
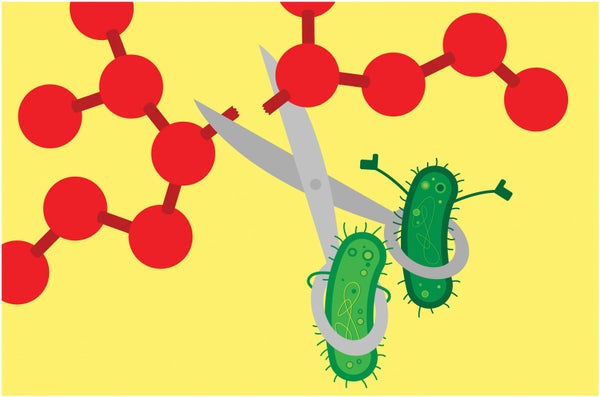
A group of bacteria has proved adept at destroying the ultratough carbon-fluorine bonds that give “forever chemicals” their name. This finding boosts hopes that microbes might someday help remove these notoriously pervasive pollutants from the environment.
Nearly 15,000 chemicals commonly found in everyday consumer products such as pizza boxes, rain jackets and sunscreens are recognized as perfluoroalkyl and polyfluoroalkyl substances, or PFASs. These chemicals can enter the body via drinking water or sludge-fertilized crops, and they have already infiltrated the blood of almost every person in the U.S. Scientists have linked even low levels of chronic PFAS exposure to myriad health effects such as kidney cancer, thyroid disease and ulcerative colitis.
Current methods to destroy PFASs require extreme heat or pressure, and they work safely only on filtered-out waste. Researchers have long wondered whether bacteria could break down the chemicals in natural environments, providing a cheaper and more scalable approach. But carbon-fluorine bonds occur mainly in humanmade materials, and PFASs have not existed long enough for bacteria to have specifically evolved the ability to digest them. The new study—though not the first to identify a microbe that destroys carbon-fluorine bonds—provides a step forward, says William Dichtel, a chemist at Northwestern University who studies energy-efficient ways to chemically degrade PFASs.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
To identify a promising set of bacteria, the study’s authors screened several microbe communities living in wastewater. Four strains from the Acetobacterium genus stood out, the team reported in Science Advances. Each strain produced an enzyme that can digest caffeate—a naturally occurring plant compound that roughly resembles some PFASs. This enzyme replaced certain fluorine atoms in the PFASs with hydrogen atoms; then a “transporter protein” ferried the fluoride ion by-products out of the single-celled microbes, protecting them from damage. Over three weeks most of the strains split the targeted PFAS molecules into smaller fragments that could be degraded more easily via traditional chemical means.
By directly targeting carbon-fluorine bonds, the Acetobacterium bacteria partially digested perfluoroalkyls, a type of PFAS that very few microbes can break down. Even so, these Acetobacterium strains could work only on perfluoroalkyl molecules that contain carbon-carbon double bonds adjacent to the carbon-fluorine ones. These “unsaturated” perfluoroalkyl compounds serve as building blocks for most larger PFASs; they are produced by chemical manufacturers and also emerge when PFASs are destroyed via incineration.
Scientists had previously demonstrated that a microbe called Acidimicrobium sp. strain A6 could break down carbon-fluorine bonds and completely degrade two of the most ubiquitous perfluoroalkyls. This microbe grows slowly, however, and requires finicky environmental conditions to function. And researchers do not yet fully understand how this bacterial strain does the job.
The Acetobacterium lines target a separate group of PFASs, and the team hopes to engineer the microbes to either improve their efficiency or expand their reach—potentially to more perfluoroalkyls. Lead study author Yujie Men of the University of California, Riverside, imagines the microbes would perform best in combination with other approaches to degrade PFASs. The range of chemical structures in these compounds means “a single lab cannot solve this problem.”
Any future commercial use of the microbes would face numerous hurdles, including breakdown speed and replicability outside of the lab, but Men looks forward to seeing how far her team can push the technique. “We’re paving the road as we go,” she says with a laugh.